History of CRISPR Technology
The history of CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) technology traces back to the early 1990s when scientists first noticed repetitive DNA sequences in the genomes of certain bacteria. However, it wasn’t until 2005 that the true potential of CRISPR was realized.
It was in 2005 that researchers at the University of Alicante, Spain, and the University of Nottingham, UK, independently discovered that these repetitive sequences were actually a bacterial defense mechanism against viral infections. They found that bacteria used the CRISPR system to store snippets of viral DNA, known as “spacer sequences,” which acted as a genetic memory of past encounters with viruses.
Then, in 2012, biochemist Jennifer Doudna and microbiologist Emmanuelle Charpentier teamed up to make a groundbreaking discovery. They identified a molecule known as Cas9, an RNA-guided protein that acts as a “molecular scissors” capable of precisely cutting DNA. This discovery laid the foundation for CRISPR gene editing.
The next major leap in CRISPR technology occurred when Feng Zhang and his team at the Broad Institute of MIT and Harvard demonstrated the use of CRISPR-Cas9 for targeted gene editing in human cells. This achievement was published in Science in 2013 and marked a turning point in genetic engineering, making CRISPR a household name in the scientific community.
Since then, CRISPR technology has exploded in popularity, becoming the go-to tool for genetic manipulation due to its simplicity, versatility, and cost-effectiveness. In just a few short years, CRISPR has revolutionized the field of genetic research and opened up a world of possibilities for gene editing in various applications.
The rapid progress of CRISPR technology has led to significant advancements in our understanding of genetics, disease treatment, agriculture, and much more. Scientists continue to explore and refine this powerful tool, pushing the boundaries of what is possible in the realm of genetic engineering.
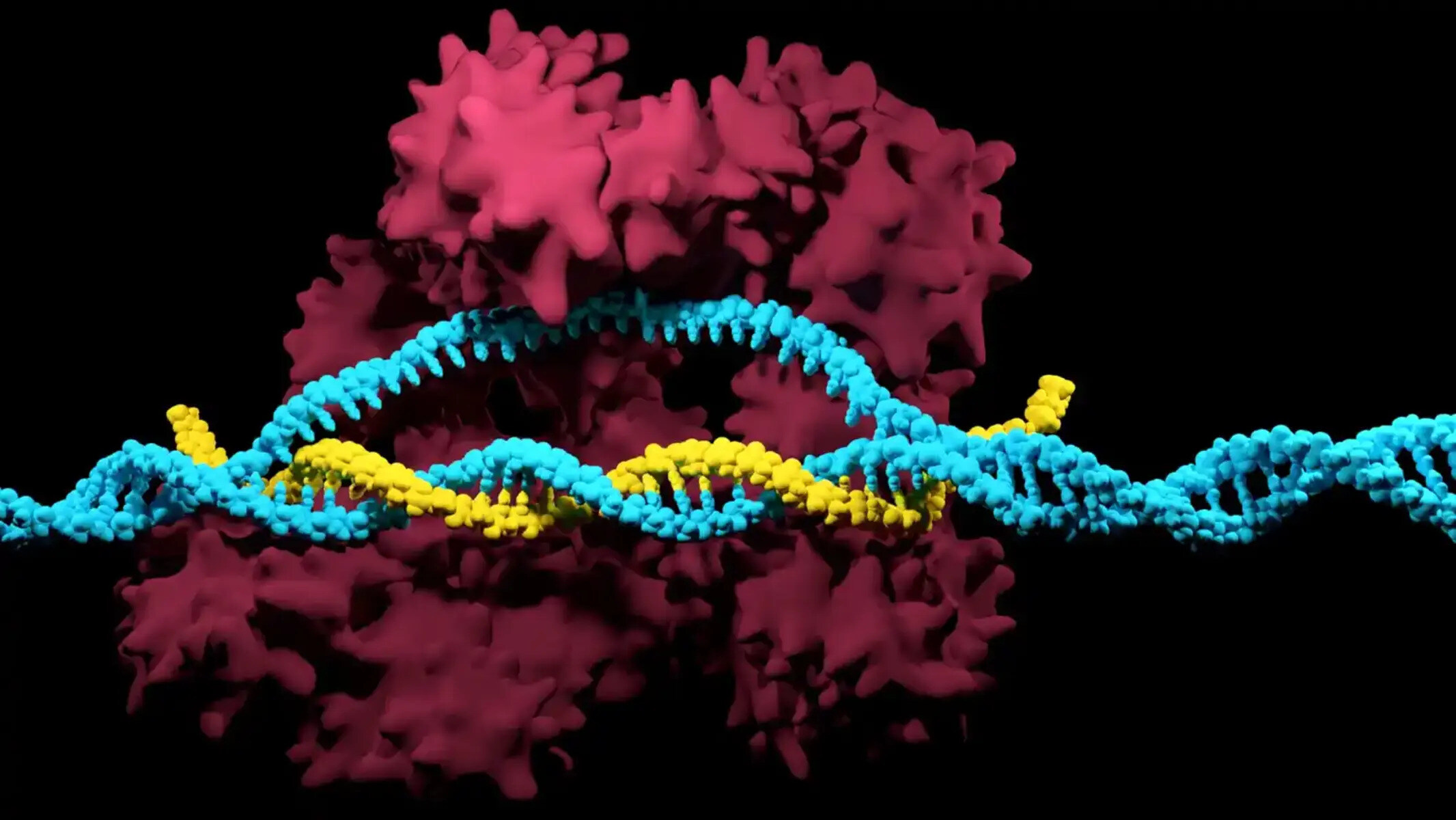
Understanding the Basics of CRISPR
CRISPR technology is a powerful tool for gene editing that allows scientists to make precise changes to an organism’s DNA. To understand how CRISPR works, let’s break it down into its basic components.
At its core, CRISPR is made up of two main components: the CRISPR array and the Cas proteins. The CRISPR array consists of repetitive DNA sequences interspaced with unique DNA segments called spacers. These spacers serve as a genetic memory of past viral infections.
The Cas proteins, specifically Cas9, play a crucial role in the CRISPR system. Cas9 acts as a pair of molecular scissors that can cut the DNA at a specific location. The key to guiding Cas9 to the desired location is a small piece of RNA called the guide RNA (gRNA), which is complementary to the target DNA sequence.
The CRISPR process begins when the CRISPR array detects a foreign DNA sequence, usually from a virus. Once identified, the CRISPR array transcribes the spacer sequence into the gRNA, which then binds to the Cas9 protein. The Cas9-gRNA complex scans the organism’s genome for a DNA sequence that matches the gRNA, and once a match is found, Cas9 cuts the DNA at that location.
After the DNA is cut, the organism’s natural repair mechanisms kick in. These repair mechanisms can lead to one of two outcomes. First, the DNA can be repaired by joining the ends together, resulting in the original sequence being restored. Alternatively, scientists can introduce a template DNA sequence during the repair process, allowing them to insert new genetic material or make specific changes to the DNA sequence.
It’s important to note that, while the CRISPR system is highly precise, off-target effects can occur, where unintended DNA sequences are altered. Researchers are continuously working on improving the specificity of the CRISPR system to minimize off-target effects.
Overall, CRISPR technology provides a relatively simple and efficient method for precise gene editing. By understanding the basics of CRISPR, scientists can harness this revolutionary tool to unlock new insights into genetics, develop innovative therapies, and potentially reshape the future of medicine and beyond.
Components of CRISPR System
The CRISPR system consists of several key components that work together to enable precise gene editing. Understanding these components is crucial to comprehending how CRISPR technology functions. Let’s delve into the various elements of the CRISPR system.
1. CRISPR Array: The CRISPR array serves as a storage system for viral DNA fragments, acting as the genetic memory of past viral infections. It is comprised of repetitive DNA sequences interspersed with unique spacer sequences.
2. Guide RNA (gRNA): The guide RNA is a small piece of RNA that guides the CRISPR-associated endonuclease (usually Cas9) to the target DNA sequence. The gRNA is engineered to be complementary to a specific target sequence within the genome.
3. CRISPR-Associated Endonuclease (Cas): Cas proteins are enzymes that are responsible for cutting the DNA at the desired location. Cas9 is the most widely used Cas protein in CRISPR technology due to its simplicity and efficiency.
4. Protospacer Adjacent Motif (PAM): PAM is a short DNA sequence located near the target DNA sequence. It acts as a recognition site for the Cas protein. Different Cas proteins have specific PAM sequences they recognize.
5. Target DNA Sequence: The target DNA sequence is the specific section of DNA that scientists want to modify or edit. It is identified by the gRNA and serves as the site where the Cas protein will create a double-stranded DNA break.
6. Repair Mechanisms: After the Cas protein cuts the DNA at the target sequence, the organism’s natural repair mechanisms come into play. Two common repair pathways are non-homologous end joining (NHEJ) and homology-directed repair (HDR). NHEJ rejoins the cut ends together, often resulting in small insertions or deletions (indels). HDR utilizes an introduced template DNA sequence to make specific changes or insertions into the genome.
7. Off-Target Effects: While CRISPR technology is highly precise, off-target effects can occur, where unintended DNA sequences are altered. These off-target effects can be minimized by designing gRNAs with high specificity or by using modified Cas proteins.
By understanding the components of the CRISPR system, scientists can design experiments and optimize the gene editing process to achieve accurate and targeted modifications in various organisms.
Gene Editing with CRISPR
CRISPR technology has revolutionized the field of gene editing, providing scientists with a precise and efficient tool to make targeted modifications to an organism’s DNA. Let’s delve into how gene editing is accomplished using CRISPR.
The process of gene editing with CRISPR begins by designing a guide RNA (gRNA) that is complementary to the target DNA sequence. The gRNA is engineered to guide the CRISPR-associated endonuclease, most commonly Cas9, to the desired location in the genome.
Once the gRNA binds to the target sequence, Cas9 acts as a molecular scissors and cuts the DNA at that location. This creates a double-stranded break (DSB) in the DNA molecule.
After the DSB is created, the organism’s natural repair mechanisms spring into action. Two primary repair pathways are non-homologous end joining (NHEJ) and homology-directed repair (HDR).
NHEJ is the default repair pathway and rejoins the cut ends of the DNA together. However, this repair mechanism often introduces small insertions or deletions (indels) at the site of the break, leading to gene disruption or loss of function. NHEJ is commonly used when the goal is to disrupt a gene or create small insertions/deletions.
HDR, on the other hand, requires the presence of a template DNA sequence that is homologous to the regions surrounding the DSB. This template DNA can be introduced along with the CRISPR-Cas system and can be used to insert new genetic material or make specific changes to the DNA sequence.
The ability to precisely edit the genome using CRISPR has immense potential for various applications. It can be used to study the function of specific genes by disrupting them, correct genetic mutations responsible for inherited diseases, or engineer traits in plants and animals.
Furthermore, the development of advanced CRISPR techniques, such as base editing and prime editing, has expanded the scope of gene editing possibilities. Base editing enables precise changes to single nucleotides without creating DSBs, and prime editing provides even more flexibility in editing DNA sequences.
However, it is important to note that gene editing with CRISPR is a complex process that requires careful planning and consideration. Ethical and safety considerations must be taken into account when applying CRISPR technology to living organisms.
Applications of CRISPR Technology
The development of CRISPR technology has unlocked a wide range of applications across various fields. Let’s explore some of the key areas where CRISPR is being applied.
1. Human Gene Therapy: CRISPR has the potential to revolutionize the treatment of genetic diseases. By correcting or editing disease-causing mutations in the human genome, CRISPR-based therapies offer new hope for individuals suffering from inherited conditions such as cystic fibrosis, sickle cell anemia, and muscular dystrophy.
2. Cancer Treatment: CRISPR technology is being explored as a tool to target and disrupt cancer-causing genes. By specifically targeting cancer cells, CRISPR has the potential to make traditional cancer treatments more effective and minimize harm to healthy cells.
3. Agriculture and Food: CRISPR can accelerate advancements in agriculture by improving crop traits such as disease resistance, yield, and nutritional content. This technology can help develop crops that require fewer pesticides, are more resilient to changing environmental conditions, and have enhanced nutrient profiles.
4. Animal Breeding and Livestock: CRISPR offers a precise and efficient method for modifying animal genomes. This can lead to the development of animals with desirable traits such as increased milk production, disease resistance, and improved meat quality. Additionally, CRISPR can be used to combat diseases that affect livestock, improving animal welfare and reducing economic losses.
5. Biomedical Research: CRISPR has become an indispensable tool for biomedical research. It allows scientists to study the functions of specific genes, unravel disease mechanisms, and develop more accurate models for human diseases. CRISPR also enables the creation of genetically modified animal models, leading to a greater understanding of fundamental biological processes.
6. Environmental Conservation: CRISPR can contribute to environmental conservation efforts. It can be used to modify the genomes of pests or invasive species, potentially reducing their population sizes and minimizing ecological damage. Additionally, CRISPR can help develop strategies for environmental remediation, such as using bacteria to degrade pollutants.
7. Bacterial and Viral Detection: CRISPR-based diagnostic tools are being developed to detect and identify bacterial and viral pathogens. These tools leverage the sequence-specific nature of CRISPR to provide rapid and accurate detection, improving disease surveillance and public health response.
The applications of CRISPR technology continue to expand, and ongoing research and technological advancements are paving the way for groundbreaking uses in various industries. With careful consideration of ethical and safety implications, CRISPR has the potential to reshape medicine, agriculture, and the overall understanding of genetics.
Ethical Considerations of CRISPR
As CRISPR technology continues to advance, it is essential to address the ethical implications associated with its applications. Here are some of the key ethical considerations raised by the use of CRISPR.
1. Off-Target Effects: Ensuring the precision of CRISPR-mediated gene editing is crucial. The potential for unintended mutations in off-target regions raises concerns about the potential long-term effects on an organism’s health and genetic stability. Researchers are actively working to minimize off-target effects and improve the specificity of CRISPR systems.
2. Germline Editing: The ability to edit the germline, which refers to the DNA passed on to future generations, raises ethical questions. Germline editing could potentially alter the human gene pool, presenting ethical dilemmas surrounding safety, consent, and the potential for unintended consequences in future generations.
3. Human Enhancement: CRISPR technology opens up the possibility of enhancing human characteristics beyond therapeutic purposes. The potential to modify non-disease related traits, such as intelligence or physical abilities, raises concerns about exacerbating social inequalities and the creation of a “designer baby” phenomenon.
4. Informed Consent: When applying CRISPR technology in human subjects, obtaining informed consent becomes crucial. Clear communication about the potential risks, benefits, and limitations of the procedure is essential to ensure individuals can make autonomous and informed decisions regarding their own genetic information.
5. Equitable Access: Widespread adoption of CRISPR technology raises concerns about equitable access to the benefits it offers. Ensuring that these tools and treatments are accessible to all, regardless of socio-economic status or geographic location, is crucial to avoid exacerbating existing disparities in healthcare and technological access.
6. Environmental Impact: Altering the genetic makeup of organisms through gene editing can have ecological consequences. The release of genetically modified organisms into the environment raises concerns about potential ecological disruption, unintended effects on non-target species, or the creation of genetically modified organisms that could inadvertently outcompete or dominate natural populations.
7. Regulation and Oversight: Developing appropriate regulations and oversight mechanisms is essential to ensure responsible and ethical use of CRISPR technology. Balancing scientific progress with ethical considerations can help navigate potential risks and ensure that CRISPR applications align with ethical norms and societal values.
Addressing these ethical considerations requires a collaborative approach involving scientists, policymakers, ethicists, and the public. Open dialogue and ongoing ethical discussions will help guide the responsible development and use of CRISPR technology, fostering trust, transparency, and accountability in its applications.
Challenges and Limitations of CRISPR Technology
While CRISPR technology holds immense potential, it also faces several challenges and limitations that need to be addressed for its widespread application. Here are some of the key challenges associated with CRISPR technology:
1. Off-Target Effects: One of the major challenges of CRISPR technology is the occurrence of off-target effects, where unintentional modifications occur in regions of the genome that were not targeted. These off-target effects can introduce unintended genetic changes, potentially leading to unforeseen consequences. Ongoing research focuses on improving the specificity of CRISPR systems to minimize off-target effects.
2. Delivery and Efficiency: Getting the CRISPR components, including Cas enzymes and guide RNA, into the target cells efficiently remains a challenge. The delivery systems used to introduce CRISPR components into cells must be optimized to ensure effective gene editing. Additionally, efficiency can vary across different cell types and organisms, limiting its broader application.
3. Large DNA Modifications: While CRISPR technology is effective at making small changes in the DNA sequence, making larger modifications, such as inserting whole genes or deleting large sections of DNA, can be more challenging. Precisely manipulating large DNA fragments with high efficiency remains an area of active research.
4. Off-Target RNA Interactions: Recent research has revealed that guide RNA molecules can interact with unintended RNA targets, leading to potential off-target effects at the RNA level. Understanding and minimizing these off-target RNA interactions is crucial for ensuring the safety and accuracy of CRISPR applications.
5. Ethical and Regulatory Frameworks: The ethical considerations associated with CRISPR technology, such as germline editing and equitable access to gene therapies, present challenges in developing appropriate regulations and oversight. Establishing robust ethical and regulatory frameworks that promote responsible use and address societal concerns is essential.
6. Delivery to Specific Tissues: Delivering CRISPR components to specific tissues or organs within a living organism remains a challenge. Efficient delivery strategies that target specific tissues while minimizing off-target effects are necessary for therapeutic applications in various diseases.
7. Immune Response: The introduction of foreign molecules into cells, such as the Cas proteins, can trigger an immune response, leading to potential safety concerns and reduced efficiency. Understanding and mitigating immune responses to CRISPR components are important for successful gene editing.
Despite these challenges, ongoing research and advancements in CRISPR technology continue to address these limitations. Further investigations and innovations are needed to overcome these hurdles and unlock the full potential of CRISPR technology in diverse scientific and medical applications.
CRISPR vs Other Gene Editing Techniques
CRISPR technology has revolutionized gene editing, but it is important to understand how it compares to other gene editing techniques. Here, we explore the key differences between CRISPR and some of the traditional gene editing methods.
1. Zinc Finger Nucleases (ZFNs): ZFNs are engineered proteins that can be designed to target specific DNA sequences. Like CRISPR, ZFNs create double-strand breaks in the DNA. However, CRISPR offers several advantages over ZFNs, including ease of design and higher efficiency in creating targeted genetic modifications.
2. Transcription Activator-Like Effector Nucleases (TALENs): TALENs are similar to ZFNs in their ability to target specific DNA sequences and induce double-strand breaks. However, they require more complex design and construction processes compared to CRISPR. CRISPR’s simplicity and versatility have made it the preferred gene editing tool in many research and therapeutic applications.
3. Homologous Recombination (HR): HR is a natural DNA repair mechanism that can be harnessed for targeted gene editing. CRISPR offers a more efficient and precise alternative to HR-based gene editing. While HR requires the introduction of a DNA repair template, CRISPR’s ability to induce DNA breaks enhances the efficiency of gene editing, especially in non-dividing cells.
4. Meganucleases: Meganucleases are enzymes that can be engineered to recognize specific DNA sequences and induce double-strand breaks. Compared to CRISPR, meganucleases have more limited target sequence options and can be more challenging to design and engineer. CRISPR’s flexibility in target selection and simpler design has made it more widely adopted.
5. Adenine Base Editors (ABEs) and Cytosine Base Editors (CBEs): Base editing technologies, such as ABEs and CBEs, offer a distinct advantage over CRISPR in their ability to make specific single-nucleotide changes without creating double-strand breaks. However, base editors are limited to specific types of DNA modifications, whereas CRISPR offers more versatile editing capabilities.
Compared to these traditional gene editing techniques, CRISPR is generally considered superior due to its simplicity, versatility, and higher efficiency in inducing targeted genetic modifications. CRISPR’s ability to precisely edit genes, disrupt gene function, and introduce specific changes in DNA sequences has accelerated advancements in genetic research and therapeutic applications.
However, it’s worth noting that different gene editing techniques may have their own specific advantages and contexts for use, and researchers continue to explore and refine these methods to address specific research or therapeutic goals. Ultimately, the choice of gene editing technique depends on the specific objectives, target sequences, and desired outcomes in a given study or application.
CRISPR in Medicine and Healthcare
CRISPR technology holds tremendous promise for revolutionizing the field of medicine and healthcare. Here, we highlight some of the key applications of CRISPR in improving human health.
1. Gene Therapy: CRISPR technology offers the potential to treat genetic disorders by correcting or modifying disease-causing genes. By precisely editing the patient’s genome, CRISPR can target and correct specific mutations responsible for inherited diseases such as cystic fibrosis, sickle cell anemia, and muscular dystrophy. Gene therapies based on CRISPR are undergoing clinical trials and show great promise in providing long-lasting treatment for these previously incurable conditions.
2. Cancer Treatment: CRISPR technology has the potential to revolutionize cancer treatment by directly targeting cancer cells and disrupting the genes responsible for tumor growth. By precisely editing the cancer genome, CRISPR can inhibit the expression of oncogenes, activate tumor suppressor genes, or enhance the effectiveness of existing therapies. Responsive and customizable cancer treatments based on CRISPR offer new possibilities for more effective and personalized cancer therapies.
3. Infectious Disease Management: CRISPR-based approaches can aid in the management of infectious diseases. CRISPR can be used to target and eliminate specific viral sequences, potentially providing a breakthrough in the treatment or prevention of viral infections such as HIV, hepatitis B, or respiratory viruses. CRISPR-based diagnostics are also being developed to rapidly detect and identify infectious pathogens.
4. Drug Development: CRISPR technology facilitates the study of disease mechanisms, allowing researchers to identify and validate therapeutic targets for drug development. By creating precise disease models, CRISPR accelerates the discovery of new treatments and therapies for a wide range of diseases, including neurodegenerative disorders, cardiovascular diseases, and autoimmune conditions.
5. Organ Transplantation: CRISPR may offer solutions to the organ transplantation shortage by enabling xenotransplantation, the transplantation of organs from genetically modified animals into humans. CRISPR can be used to modify the genes of pigs or other animals to reduce the risk of organ rejection and make them suitable donors for human transplantation, potentially addressing the critical shortage of organs for transplantation.
6. Precision Medicine: CRISPR technology enables personalized medicine by facilitating the development of patient-specific treatments. Genome editing can be tailored to individual patients, allowing for precise modifications based on their unique genetic backgrounds. This personalized approach has the potential to significantly improve treatment outcomes and reduce adverse effects.
Overall, CRISPR technology is poised to transform medicine and healthcare by providing powerful tools for precise gene editing, disease modeling, and targeted therapies. While challenges and ethical considerations exist, ongoing research and improvements in CRISPR technology continue to drive advancements in improving human health and well-being.
CRISPR in Agriculture and Food
CRISPR technology offers immense potential to revolutionize agriculture and food systems, addressing challenges related to crop productivity, climate change, and sustainable food production. Here, we explore some of the applications of CRISPR in agriculture and food.
1. Enhanced Crop Traits: CRISPR technology can be used to improve crop traits such as disease resistance, tolerance to specific environmental conditions, and enhanced nutritional content. By editing the genes responsible for these traits, CRISPR enables the development of crops that require fewer pesticides, are more resilient to climate change, and have improved nutrient profiles.
2. Disease and Pest Management: CRISPR can help develop crop varieties with enhanced resistance against diseases, pests, and pathogens. By modifying the genes involved in plant-pathogen interactions, CRISPR can confer natural resistance, reducing the need for chemical pesticides and promoting sustainable agriculture practices.
3. Climate Adaptation: Climate change poses significant challenges to agriculture. CRISPR technology can aid in developing climate-resilient crops by introducing genetic modifications that enhance drought tolerance, heat resistance, or adaptability to changing environmental conditions. This can help safeguard crop yields and maintain food security in the face of climate variability.
4. Improved Yield and Quality: By targeting genes associated with crop yield and quality, CRISPR can contribute to increasing agricultural productivity. Editing genes involved in plant growth, yield-related traits, and nutritional composition can lead to superior crop varieties with improved productivity, nutritional value, and post-harvest storability.
5. Reduced Food Waste: CRISPR technology can help reduce food waste by improving the shelf life of agricultural produce. By modifying genes involved in ripening and decay, CRISPR can extend the post-harvest life of fruits and vegetables, reducing spoilage and losses in the supply chain.
6. Environmental Impact: CRISPR can contribute to environmental conservation efforts in agriculture. By developing crops with higher resource-use efficiency, reduced environmental footprint, and enhanced sustainability, CRISPR-based agriculture practices can minimize negative impacts on land, water, and biodiversity.
7. Precision Breeding: CRISPR technology enables precision breeding, accelerating the development of new crop varieties with desired traits. Compared to traditional breeding methods, which rely on crossing and selection over multiple generations, CRISPR allows targeted modifications in a shorter time frame, expediting the development of improved crop varieties.
CRISPR technology has the potential to revolutionize agriculture, promoting sustainable practices, improving crop productivity, and addressing global food security challenges. However, it is crucial to consider potential regulatory frameworks, ethical concerns, and public acceptance to ensure the responsible and beneficial use of CRISPR in agriculture and food systems.
CRISPR in Environmental ConservationCRISPR technology holds promise for advancing environmental conservation efforts, providing new tools and strategies for addressing pressing challenges in biodiversity conservation, ecosystem restoration, and sustainable resource management. Here, we delve into some of the applications of CRISPR in environmental conservation.
1. Conservation Genetics: CRISPR can aid in the conservation of endangered species by enabling precise genetic interventions. By editing genes associated with disease resistance, reproductive fitness, or immune responses, CRISPR can potentially improve the resilience and survival of endangered populations, contributing to their conservation.
2. Invasive Species Management: Invasive species pose a significant threat to biodiversity and ecosystem functioning. CRISPR can be utilized to develop gene-drive systems that can restrict the reproduction or alter the genetic traits of invasive species. This holds the potential to control or eradicate invasive populations, restoring ecological balance and protecting native flora and fauna.
3. De-extinction: CRISPR may offer opportunities for de-extinction efforts, aiming to revive extinct species. Through genetic engineering, CRISPR can potentially reintroduce genes from extinct species into closely related living species, resurrecting genetic traits lost to extinction and restoring ecological functions and biodiversity.
4. Environmental Monitoring and Remediation: CRISPR-based tools can be used for environmental monitoring and assessment. CRISPR-based biosensors can detect and identify specific pollutants or contaminants, providing real-time monitoring and assessment of environmental quality. CRISPR-mediated gene editing in microorganisms can also be harnessed for environmental remediation, such as breaking down pollutants or enhancing bioremediation capabilities.
5. Conservation Breeding Programs: CRISPR holds potential for improving the efficiency and success of conservation breeding programs. It can be used to enhance the genetic diversity of captive populations, reducing inbreeding and increasing the genetic fitness of individuals. Precise genome editing can also help address genetic diseases and promote adaptive traits in managed populations, supporting their long-term viability.
6. Habitat Restoration: CRISPR technology can contribute to habitat restoration efforts by modifying plants or microorganisms involved in ecosystem rehabilitation. By enhancing the growth, adaptability, or stress tolerance of native species, CRISPR can aid in the restoration of degraded habitats, promoting ecological recovery and enhancing resilience.
Applying CRISPR in environmental conservation is an evolving field that requires careful consideration of ethical, legal, and ecological implications. Balancing conservation goals with ecosystem integrity and potential unintended consequences will be essential in harnessing CRISPR’s potential as a tool for environmental conservation and preserving biodiversity.
CRISPR in Biotechnology and Research
CRISPR technology has transformed the landscape of biotechnology and research, offering powerful tools for studying gene function, advancing scientific discoveries, and driving innovation. Here, we explore some of the key applications of CRISPR in biotechnology and research.
1. Functional Genomics: CRISPR technology enables researchers to study gene function by systematically disrupting or modifying specific genes in model organisms. This approach helps elucidate the roles of genes in biological processes, diseases, and development. CRISPR-based knockout libraries have become invaluable resources for large-scale gene function studies.
2. Drug Discovery and Development: CRISPR facilitates the identification and validation of potential drug targets. By using CRISPR to introduce precise genetic modifications, researchers can study the effects of gene alterations on disease processes, identifying new therapeutic targets or testing drug efficacy in disease models. CRISPR also aids in developing more accurate disease models for drug screening.
3. Bioproduction and Biomanufacturing: CRISPR-based gene editing can optimize microbial hosts for industrial bioproduction. This includes engineering microorganisms to produce valuable products such as pharmaceuticals, biofuels, and biochemicals with higher yields and improved properties. CRISPR also enables the targeted engineering of metabolic pathways to enhance productivity and create novel bio-based products.
4. Gene Therapy Development: CRISPR has revolutionized the field of gene therapy by offering precise tools to correct disease-causing mutations. Researchers can use CRISPR to edit patient-specific cells ex vivo, correcting genetic defects before reinfusing them into the patient. CRISPR technology also aids in developing viral vectors for gene delivery as part of gene therapy approaches.
5. Stem Cell Engineering: CRISPR enables the manipulation of stem cells for regenerative medicine and tissue engineering. By editing specific genes, researchers can direct stem cells to differentiate into desired cell types or repair genetic mutations in patient-specific pluripotent stem cells, opening up new possibilities for personalized medicine and cell-based therapies.
6. Genome-Wide Screens: CRISPR-based screening techniques allow researchers to systematically interrogate large numbers of genes simultaneously. This enables the identification of genes involved in specific biological processes, diseases, or drug responses. CRISPR screening has greatly advanced our understanding of cellular pathways and disease mechanisms.
7. Synthetic Biology: CRISPR technology plays a critical role in synthetic biology, allowing the construction of synthetic gene circuits and the engineering of novel biological systems. CRISPR enables precise genome editing and DNA manipulation, facilitating the construction of genetic circuits for biosensors, biofuels, and other synthetic biology applications.
CRISPR technology continues to push the boundaries of biotechnology and research, enabling breakthroughs in a wide range of fields. Its versatility, efficiency, and precision have accelerated scientific discoveries, opened new possibilities, and paved the way for innovative applications with far-reaching implications.
Future Potential of CRISPR Technology
The future potential of CRISPR technology is vast, with ongoing advancements and discoveries shaping its trajectory. Here, we explore some of the key areas where CRISPR is poised to have a significant impact in the coming years.
1. Therapeutic Applications: CRISPR-based gene therapies have the potential to revolutionize the treatment of genetic diseases and inherited disorders. As researchers continue to refine CRISPR techniques and improve delivery methods, we can expect to see more clinical trials and advancements in using CRISPR for precise and personalized therapeutics.
2. Precision Agriculture: In agriculture, CRISPR holds tremendous promise for developing climate-resilient crops, enhancing yield and nutritional content, and addressing food security challenges. As researchers discover more about genes responsible for desirable traits, CRISPR can help optimize crop production and contribute to sustainable and resource-efficient agricultural practices.
3. Engineering Ecosystems: CRISPR-enabled technologies, such as gene drives, offer the potential to engineer ecosystems and address complex environmental challenges. By targeting invasive species and mitigating the impact of human activities, CRISPR can help restore biodiversity and ecological balance, promoting environmental conservation and restoration efforts.
4. Regenerative Medicine: CRISPR’s ability to modify stem cells and correct genetic defects offers tremendous potential in regenerative medicine, tissue engineering, and organ transplantation. Researchers are working towards refining and expanding CRISPR techniques to regenerate damaged tissues, repair genetic mutations, and develop artificial organs, paving the way for transformative advancements in healthcare.
5. Non-Human Applications: CRISPR technology is not limited to human applications. Its potential extends to non-human organisms, such as engineering plants for sustainable biofuels, modifying livestock for improved productivity and disease resistance, and wildlife conservation efforts. These applications can have wide-ranging impacts on various sectors, from energy to biodiversity conservation.
6. Advancements in CRISPR Tools: Researchers are continuously working to improve CRISPR technology and overcome its limitations. This includes enhancing precision, minimizing off-target effects, and developing new functionalities such as base editing and prime editing. Future advancements in CRISPR tools and techniques will help maximize efficiency, expand target sequences, and refine editing capabilities for a broader range of applications.
7. Ethical and Societal Discussions: The continued progress of CRISPR technology calls for ongoing ethical, legal, and societal discussions. As CRISPR’s applications expand, discussions surrounding issues like equity, affordability, safety, accessibility, and responsible use become increasingly important to ensure that the benefits of CRISPR are harnessed in a manner that aligns with societal values and respects individual autonomy.
While challenges remain and ethical considerations must be addressed, the future potential of CRISPR technology is promising. Through continued research, innovation, and collaboration, CRISPR has the potential to revolutionize medicine, agriculture, conservation, and more, contributing to a brighter and healthier future.